What Is Driving Global Medical Grade 2,2,2-Trifluoroethanol Market from USD 32.5 Million in 2026 to USD 58.9 Million by 2034?
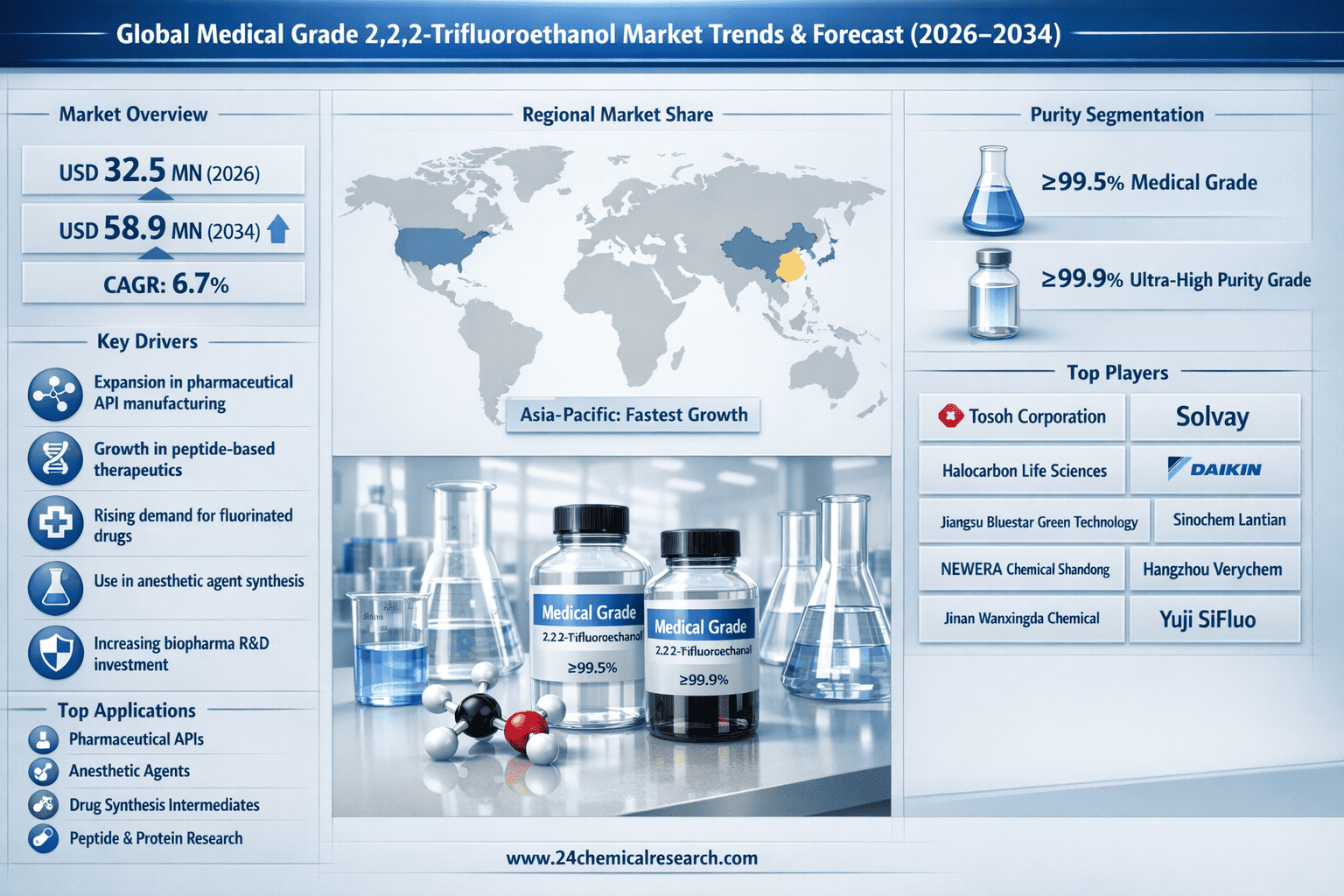
According to 24Chemical Research, Global Medical Grade 2,2,2-Trifluoroethanol Market was valued at USD 32.5 million in 2026 and is projected to reach USD 58.9 million by 2034, exhibiting a steady CAGR of 6.7% during the forecast period.
Medical Grade 2,2,2-Trifluoroethanol (TFE), a high-purity fluorinated alcohol characterized by its exceptional solvent properties and chemical stability, has transitioned from specialized laboratory use to becoming an indispensable component in pharmaceutical manufacturing. Its unique attributes—including high polarity, low nucleophilicity, and the ability to dissolve peptides and proteins without denaturation—make it a critical reagent for numerous pharmaceutical applications. Unlike industrial-grade TFE, medical grade material meets stringent purity standards essential for pharmaceutical synthesis and formulation, ensuring compatibility with Good Manufacturing Practice (GMP) requirements and regulatory compliance.
Get Full Report Here: https://www.24chemicalresearch.com/reports/306272/medical-grade-trifluoroethanol-market
Market Dynamics:
The market's trajectory is shaped by a complex interplay of powerful growth drivers, significant restraints that are being actively addressed, and vast, untapped opportunities.
Powerful Market Drivers Propelling Expansion
-
Expansion in Pharmaceutical API Manufacturing: The integration of medical grade TFE as a solvent and intermediate in the synthesis of complex active pharmaceutical ingredients (APIs), particularly fluorine-containing drugs, represents the primary growth vector. The global pharmaceutical industry, valued at over $1.4 trillion, continuously demands high-purity solvents that enable efficient and selective chemical reactions. TFE's role is crucial in building molecular scaffolds for innovative therapeutics, including antiviral and anticancer agents. The growing pipeline of new molecular entities utilizing advanced fluorine chemistry directly correlates with increased consumption, as it facilitates reactions that are challenging with conventional solvents.
-
Advancements in Peptide-Based Therapeutics: The biopharmaceutical sector is experiencing significant growth fueled by TFE's unique properties in peptide research and production. Its ability to stabilize secondary structures makes it ideal for studying protein folding and developing peptide-based drugs, particularly in targeted cancer therapies and metabolic disorders where structural integrity is paramount. Furthermore, TFE is employed in the purification and formulation stages of these sophisticated biologics. With the global peptide therapeutics market projected to exceed $50 billion by 2027, medical grade TFE is positioned as an essential enabler of next-generation biological medicines.
-
Innovations in Anesthetic Agents: The anesthesia sector relies on TFE as a key starting material for certain inhalation anesthetics, representing a stable and consistent demand segment. Its chemical structure serves as a building block for modern fluorinated anesthetics that offer improved safety profiles and faster recovery times compared to older alternatives. The persistent global demand for surgical procedures and the development of new anesthetic formulations in emerging markets ensure continued utilization of high-purity TFE in this critical medical application.
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/306272/medical-grade-trifluoroethanol-market
Significant Market Restraints Challenging Adoption
Despite its critical applications, the market faces substantial hurdles that must be overcome to achieve broader adoption.
-
Stringent Production and Handling Requirements: The sophisticated purification processes required to produce medical grade TFE, involving multiple distillation steps and specialized equipment, elevate manufacturing costs by 25-35% above industrial-grade equivalents. These processes must be conducted in controlled environments to prevent contamination, adding significant operational expenses. The compound's classification as a hazardous material necessitates specialized storage, handling protocols, and transportation measures, increasing overall costs and complicating logistics for end-users, particularly smaller pharmaceutical companies.
-
Regulatory and Environmental Pressures: In pharmaceutical applications, the path to regulatory approval for processes utilizing novel solvents is lengthy and complex. Compliance with pharmacopeial standards (USP, EP) requires extensive documentation and validation studies, potentially extending timelines by 18-24 months. Additionally, increasing environmental, health, and safety (EHS) regulations concerning fluorinated compounds are prompting industries to seek greener alternatives. This regulatory scrutiny, particularly in European markets under REACH regulations, creates uncertainty and may limit long-term utilization in certain applications.
Critical Market Challenges Requiring Innovation
The transition from laboratory-scale use to consistent industrial supply presents its own set of technical and operational challenges. Maintaining ultra-high purity (≥99.9%) at commercial production volumes is particularly demanding, with current processes sometimes struggling with batch-to-batch consistency due to the compound's sensitivity to moisture and other contaminants. These technical hurdles necessitate substantial R&D investments in purification technologies and quality control systems.
Furthermore, the market contends with supply chain vulnerabilities related to raw material availability. Fluctuations in the supply of key fluorination precursors and specialized equipment required for TFE production can create bottlenecks. The specialized nature of transportation and storage requirements adds another layer of complexity, as the compound must be handled under specific conditions to maintain its pharmaceutical-grade quality throughout the supply chain.
Vast Market Opportunities on the Horizon
-
Growth in Contract Manufacturing and Emerging Markets: The expanding outsourcing of API manufacturing to Contract Development and Manufacturing Organizations (CDMOs) presents a significant growth avenue. These organizations require reliable supplies of high-purity solvents to serve global pharmaceutical clients, creating stable, long-term demand. Simultaneously, emerging biotech hubs in Asia-Pacific regions offer substantial opportunities as they ramp up pharmaceutical production capabilities and research activities, driving demand for specialized reagents like medical grade TFE.
-
Development of Novel Pharmaceutical Applications: Ongoing research into new therapeutic modalities, particularly in oligonucleotide synthesis and advanced drug delivery systems, opens new applications for medical grade TFE. Its unique solvent properties show promise in emerging areas such as lipid nanoparticle formulation for mRNA therapeutics and other novel drug delivery platforms. These innovative applications could significantly expand the market beyond traditional uses.
-
Strategic Industry Partnerships: The market is witnessing increased collaboration between TFE manufacturers and pharmaceutical companies to develop application-specific solutions and ensure supply chain security. These partnerships are crucial for addressing technical challenges, streamlining regulatory compliance, and developing customized purity specifications for specific pharmaceutical applications, thereby creating more stable and predictable market dynamics.
In-Depth Segment Analysis: Where is the Growth Concentrated?
By Type:
The market is segmented into ≥99.5% and ≥99.9% purity grades. ≥99.9% purity grade currently demonstrates stronger growth momentum, favored for its superior performance in critical pharmaceutical applications where even minimal impurities can affect drug efficacy and safety. This ultra-pure grade is essential for GMP-compliant manufacturing processes and is becoming the standard for new drug applications, particularly in regulated markets.
By Application:
Application segments include Anesthetic Agents, Active Pharmaceutical Ingredients (APIs), Intermediates for Drug Synthesis, and others. The API synthesis segment currently dominates market share, driven by the continuous development of new pharmaceutical compounds requiring specialized fluorination chemistry. However, the anesthetic applications segment represents a stable, established market with consistent demand patterns.
By End-User Industry:
The end-user landscape includes Pharmaceutical Companies, Contract Research & Manufacturing Organizations (CRAMOs), and Academic & Research Institutions. Pharmaceutical companies account for the majority share, leveraging TFE for large-scale production of approved drugs. Their demand is driven by the need for reliable, high-quality materials that meet strict regulatory standards. The CRAMO segment is experiencing rapid growth as outsourcing of pharmaceutical manufacturing increases globally.
Download FREE极速快3 Sample Report: https://www.24chemicalresearch.com/download-sample极速快3/306272/medical-grade-trifluoroethanol-market
Competitive Landscape:
The global Medical Grade 2,2,2-Trifluoroethanol market is semi-consolidated and characterized by specialized competition among chemical manufacturers with pharmaceutical capabilities. The market features established global players with significant expertise in fluorine chemistry, alongside specialized regional manufacturers focusing on specific market segments.
List of Key Medical Grade 2,2,2-Trifluoroethanol Companies Profiled:
-
Tosoh Corporation (Japan)
-
Solvay (Belgium)
-
Halocarbon Life Sciences (USA)
-
Jiangsu Bluestar Green Technology Co (China)
-
Daikin (Japan)
-
NEWERA CHEMICAL SHANDONG CO (China)
-
Hangzhou Verychem Science And Technology Co (China)
-
Sinochem Lantian (极速快3China)
-
Jinan Wanxingda Chemical (China)
-
Yuji SiFluo (China)
The competitive strategy focuses heavily on maintaining stringent quality控制, expanding production capabilities for high-purity grades, and developing long-term partnerships with pharmaceutical end-users. Companies are investing in R&D to improve purification processes and reduce costs while ensuring compliance with evolving regulatory requirements across different markets.
Regional Analysis: A Global Footprint with Distinct Leaders
-
North America: Is a dominant market, holding a significant share of global consumption. This leadership is fueled by a robust pharmaceutical industry, stringent regulatory standards that demand high-purity materials, and substantial R&D investment in new drug development. The presence of major pharmaceutical companies and advanced research institutions creates sustained demand for medical grade TFE.
-
Europe & Asia-Pacific: Together form a powerful secondary market bloc. Europe's strength is driven by its strong chemical industry and pharmaceutical sector, with strict adherence to quality standards. The Asia-Pacific region, particularly China, represents the fastest-growing market, supported by expanding pharmaceutical manufacturing capabilities, government support for the pharmaceutical industry, and increasing healthcare investment. The region is also becoming a significant production hub for medical grade chemicals.
-
Other Regions: South America and Middle East & Africa represent emerging markets with growing potential. These regions are witnessing increasing pharmaceutical production and healthcare investment, though they currently represent smaller portions of the global market. Their growth is driven by efforts to develop local pharmaceutical capabilities and reduce dependency on imports.
Get Full Report Here: 极速快3https://www.24chemicalresearch.com/reports/306272/medical-grade-trifluoroethanol-market
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/306272/medical-grade-trifluoroethanol-market
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
-
Plant-level capacity tracking
-
Real-time price monitoring极速快3
-
Techno-economic feasibility studies
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness