Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Industry Statistics: Growth, Share, Value, and Trends By 2032
"Global Executive Summary Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market: Size, Share, and Forecast
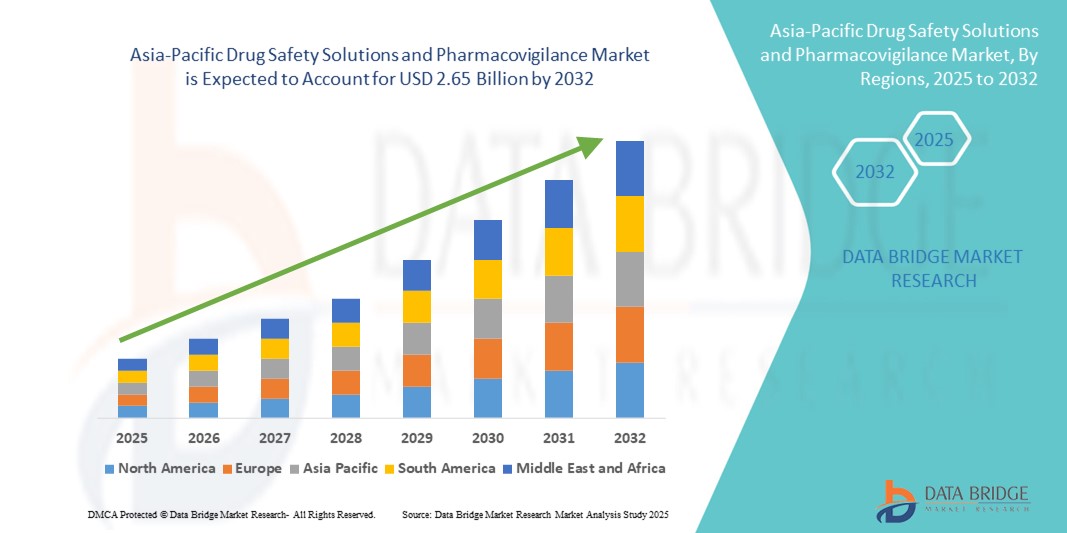
- The Asia-Pacific drug safety solutions and pharmacovigilance market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 2.65 billion by 2032, at a CAGR of 8.10% during the forecast period.
The Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market analysis report focuses on leading global industry players, providing information such as company profiles, product pictures and specifications, capacity, production, price, cost, revenue, and contact information. Geographically, this report is categorized into various main regions, including sales, proceeds, market share and expansion rate (percent) in the following areas: North America, Asia-Pacific, South America, Europe, Asia-Pacific, The Middle East and Africa. What is more, the feasibility of new investment projects is assessed and overall research conclusions are offered. Analysis of profiles of manufacturers or commanding players of the global market is performed based on sales area, key products, gross margin, revenue, price, and production.
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Analysis report analyzes the changing trends in the industry. The industry development trends and marketing channels are also analyzed. In this market document, development policies and plans are discussed, and manufacturing processes and cost structures are also analyzed. The report offers a detailed analysis of Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market industry with growth & significant CAGR during the forecast period by top manufacturer’s analysis, region, types, and market segment by applications. The market research study of this report is conducted to understand the current landscape of the global market.
Stay ahead with crucial trends and expert analysis in the latest Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report. Download now:
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Industry Overview
Segments
- Based on the type of product, the Asia-Pacific Drug Safety Solutions and Pharmacovigilance market can be segmented into integrated software solutions, database management, case processing, adverse event reporting, and others. Integrated software solutions are expected to dominate the market due to the increasing demand for efficient and streamlined pharmacovigilance processes.
- By deployment mode, the market can be classified into on-premise and cloud-based solutions. Cloud-based solutions are anticipated to witness significant growth as they offer cost-effective and scalable options for pharmacovigilance operations.
- On the basis of end-user, the market is segmented into pharmaceutical companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and others. Pharmaceutical companies are likely to hold a substantial share of the market owing to the rising emphasis on drug safety and regulatory compliance.
Market Players
- Oracle Corporation
- IQVIA
- Sparta Systems, Inc.
- United BioSource Corporation
- Cognizant
- Parexel International Corporation
- BioClinica
- Synowledge LLC
- EXTEDO
- Ennov
The Asia-Pacific Drug Safety Solutions and Pharmacovigilance market is witnessing significant growth due to factors such as the increasing focus on patient safety, stringent regulations for drug approvals, and the rising number of adverse drug reactions reported. With the adoption of advanced technologies like artificial intelligence and automation, the market is expected to experience further expansion in the coming years. Integrated software solutions are in high demand as they help streamline pharmacovigilance processes, leading to improved efficiency and better regulatory compliance. Cloud-based deployment options are gaining traction for their scalability and cost-effectiveness, especially among small and medium-sized enterprises in the region. Pharmaceutical companies are the primary end-users of drug safety solutions and pharmacovigilance services, as they strive to ensure the safety and efficacy of their products in compliance with regulatory standards. Market players like Oracle Corporation, IQVIA, and Sparta Systems, Inc. are actively investing in research and development to offer innovative solutions that cater to the evolving needs of the market.
The Asia-Pacific drug safety solutions and pharmacovigilance market is poised for continued growth driven by several key factors. One emerging trend in the market is the increasing adoption of real-time monitoring technologies to detect adverse drug reactions promptly. This shift towards proactive surveillance is essential in ensuring patient safety and regulatory compliance. Additionally, there is a growing emphasis on the integration of pharmacovigilance data with electronic health records to provide a comprehensive view of a patient's medical history and potential risk factors.
Another significant development in the market is the rise of data analytics and machine learning applications to enhance signal detection and risk assessment processes. By leveraging these advanced technologies, pharmaceutical companies and other end-users can identify potential safety concerns more efficiently and take proactive measures to mitigate risks. Moreover, the increasing complexity of clinical trials and drug development processes necessitates robust pharmacovigilance solutions that can adapt to evolving regulatory requirements and industry standards.
Furthermore, the market is witnessing a surge in strategic partnerships and collaborations among key players to expand their product offerings and geographic reach. These collaborations enable companies to leverage each other's strengths and capabilities to deliver integrated solutions that address the diverse needs of the market. By fostering innovation and knowledge sharing, these partnerships contribute to the development of cutting-edge pharmacovigilance technologies that set new industry benchmarks for safety and efficacy standards.
Moreover, as the Asia-Pacific region continues to emerge as a prominent hub for pharmaceutical research and development, the demand for advanced drug safety solutions and pharmacovigilance services is expected to grow exponentially. Market players are increasingly investing in expanding their presence in key markets within the region to capitalize on this growth opportunity. By tailoring their offerings to meet the specific requirements of local regulatory environments and healthcare systems, companies can build a competitive advantage and establish themselves as trusted partners in ensuring drug safety and regulatory compliance.
In conclusion, the Asia-Pacific drug safety solutions and pharmacovigilance market are undergoing rapid transformation driven by technological advancements, evolving regulatory landscapes, and shifting industry dynamics. Key players in the market are well-positioned to capitalize on these opportunities by investing in innovation, collaboration, and market expansion strategies. With the continued focus on patient safety and regulatory compliance, the market is set to witness sustained growth and development in the coming years, shaping the future of pharmacovigilance in the region.The Asia-Pacific drug safety solutions and pharmacovigilance market is poised for continued growth driven by various factors that are shaping the landscape of the industry in the region. One key aspect that is influencing market dynamics is the increasing focus on real-time monitoring technologies for prompt detection of adverse drug reactions. This proactive approach is crucial in ensuring patient safety and complying with stringent regulatory requirements, highlighting the importance of staying ahead in pharmacovigilance practices in the rapidly evolving healthcare landscape of the region. The integration of pharmacovigilance data with electronic health records is another trend that is gaining traction, offering a comprehensive view of patients' medical history and aiding in identifying potential risks associated with medications more effectively.
Furthermore, the incorporation of data analytics and machine learning applications is transforming signal detection and risk assessment processes within pharmacovigilance operations. By leveraging these advanced technologies, pharmaceutical companies and other stakeholders can enhance their capabilities in identifying and addressing safety concerns promptly, thereby improving the overall efficiency and effectiveness of drug safety practices. This indicates a shift towards more sophisticated and data-driven approaches in pharmacovigilance, enabling proactive risk management strategies and contributing to better patient outcomes.
In addition, strategic partnerships and collaborations among market players are playing a significant role in driving innovation and expanding market reach in the Asia-Pacific region. By joining forces, companies can leverage their collective expertise and resources to develop integrated solutions that cater to the diverse needs of the market, fostering a collaborative environment that promotes innovation and knowledge sharing. These partnerships not only enhance the capabilities of individual players but also set new industry benchmarks for safety and efficacy standards, ultimately benefiting patients and regulatory bodies in ensuring drug safety and compliance with evolving regulations.
As the Asia-Pacific region emerges as a key player in the global pharmaceutical landscape, the demand for advanced drug safety solutions and pharmacovigilance services is expected to surge significantly. Market players are strategically investing in expanding their presence in key markets within the region to capitalize on the growing opportunities presented by the evolving healthcare ecosystem. By customizing their offerings to align with local regulatory requirements and healthcare systems, companies can establish themselves as trusted partners in delivering cutting-edge pharmacovigilance solutions that meet the unique needs of the Asia-Pacific market. Overall, the market is witnessing a transformation driven by technological advancements, regulatory shifts, and industry collaborations, paving the way for sustained growth and development in the pharmacovigilance sector in the region.
Access detailed insights into the company’s market position
https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market/companies
Nucleus is Data Bridge Market Research’s cutting-edge, cloud-based market intelligence platform that empowers organizations to make faster, smarter, data-driven decisions. Designed for strategic thinkers, researchers, and innovators, Nucleus transforms complex macroeconomic indicators, industry-specific trends, and competitive data into actionable insights through dynamic dashboards and real-time analytics. With capabilities spanning market access intelligence, competitive benchmarking, epidemiological analytics, global trade insights, and cross-sector strategy modeling, the platform unifies diverse datasets to help businesses identify opportunities, assess risks, and drive growth across regions and industries. Built on a powerful neural analytics engine, Nucleus bridges the gap between raw data and strategic execution, enabling users to visualize emerging trends, benchmark performance, and make informed decisions with confidence.
Get More Detail: https://www.databridgemarketresearch.com/nucleus/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market
Alternative Research Questions for Global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Analysis
- What is the current valuation of the global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market?
- What CAGR is projected for the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market over the forecast period?
- What are the key segments analyzed in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report?
- Which companies dominate the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market landscape?
- What geographic data is covered in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market analysis?
- Who are the leading firms operating in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market?
Browse More Reports:
Global Abdominal Pain Drugs Market
Global Accelerometers Smart Roads Market
Global Acetic Acid in Food Application Market
Global Acitretin Market
Global Acquired Ichthyosis Treatment Market
Global Acrylic Monomers Market
Global Active Insulation Market
Global Acute Coronary Syndrome Market
Global Adrenocorticotropic Hormone (ACTH) Market
Global Adsorption Equipment Market
Global Advanced Structural Ceramics Market
Global Aerospace Accumulator Market
Global Aerospace Lubricant Market
Global Age-Related Macular Degeneration (AMD) Disease - Anti VEGF Market
Global Agricultural Lubricants Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness